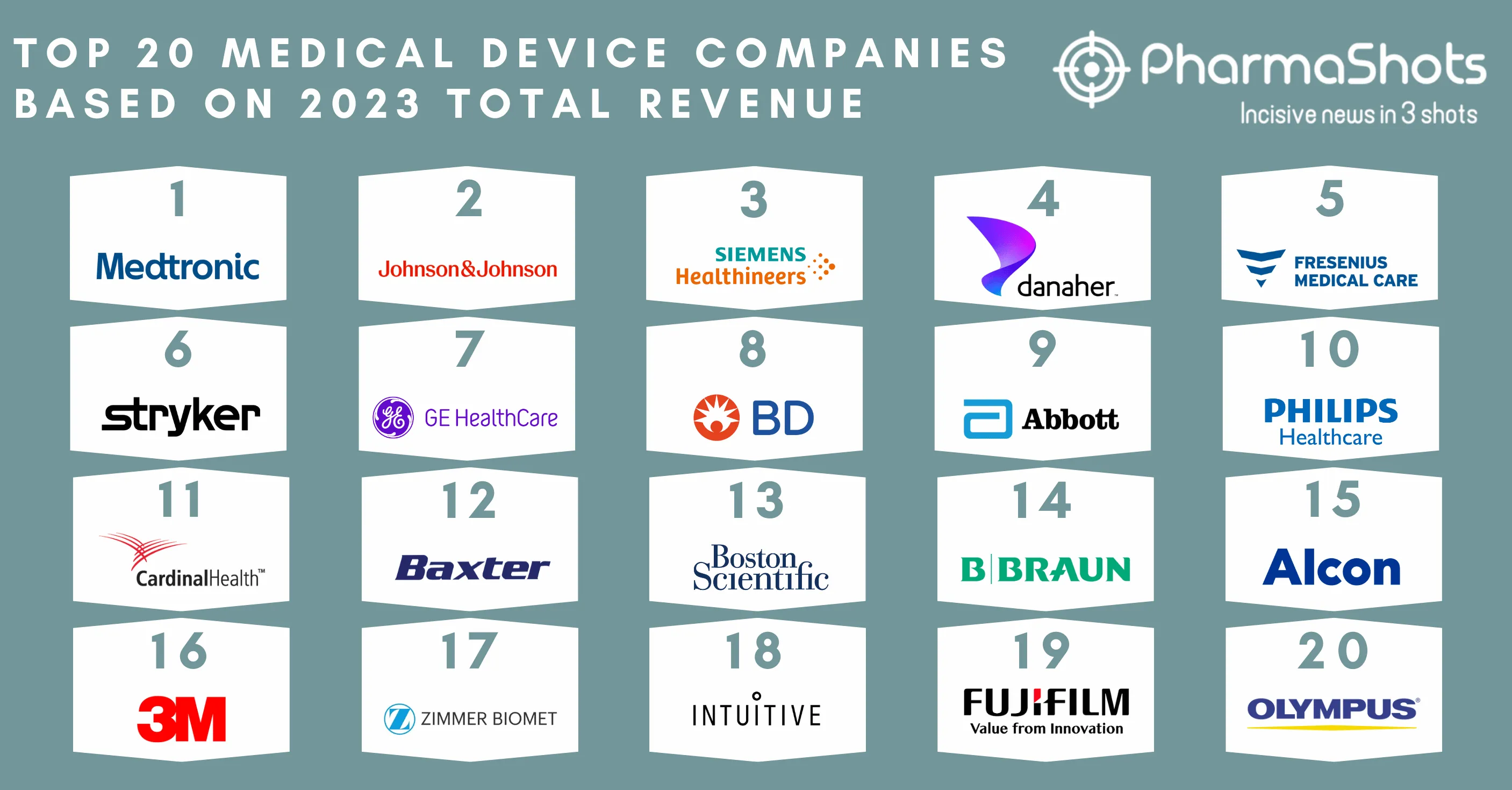
The US FDA Grants 510(k) Clearance to Zimmer Biomet’s Persona Revision SoluTion Femur
Shots:
- The US FDA has granted 510(k) clearance to Persona Revision SoluTion Femur as an alternative for knee implant in pts with metal sensitivities; commercially available in the US in Q3’25
- SoluTion Femur, part of the Persona Revision Knee System, utilizes surface-hardening treatment for enhanced wear performance & provides surgeons with anatomic components, incl. tibial as well as femoral cones & various stem choices for zonal fixation
- SoluTion Femur is made from Tivanium (Ti-6Al-4V) alloy with Ti-Nidium surface hardening for enhanced strength & is available in standard as well as plus sizes to improve flexion instability & soft tissue balancing, while reducing implant overhang
Ref: Prnewswire | Image: Zimmer Biomet
Related News:- Zimmer Biomet to Acquire Paragon 28 for ~$1.2B
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Ridhi is an avid secondary researcher who follows trends in the biopharmaceutical and healthcare sectors to curate engaging content for the global audience. She works as a news editor at PharmaShots and loves to read books and explore new destinations.